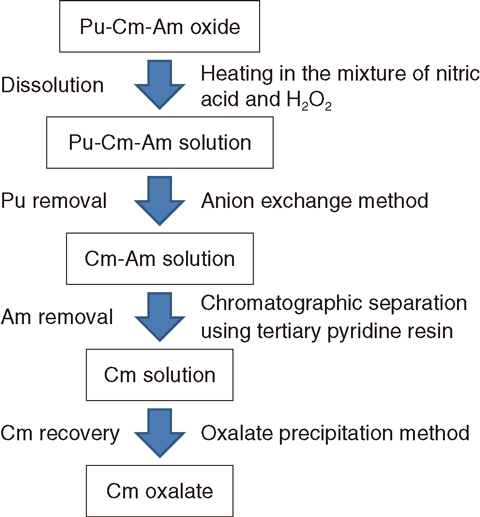
Fig.4-12 Outline of the method for preparing a high-purity curium sample from aged 244Cm oxide samples
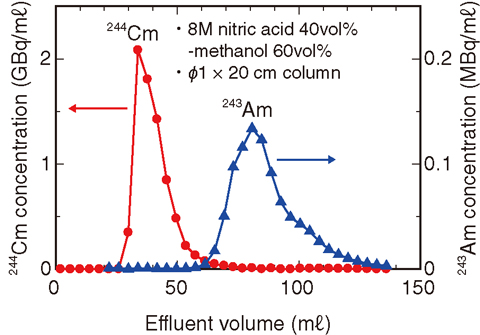
Fig.4-13 Chromatographic separation of Am and Cm using tertiary pyridine resin
Fig.4-14 The Cm oxalate sample that we prepared
Research and development of technologies for partitioning and transmutation is active in many countries including Japan. These technologies aim at reducing the burden of waste disposal by partitioning elements such as fission products and minor actinides (MA: neptunium (Np), americium (Am), curium (Cm)), which are classified as high-level wastes in the current nuclear fuel cycle, and by transmuting long-lived nuclides including MA into stable or short-lived ones. Transmutation of the long-lived nuclides is proposed to be achieved using commercial fast reactors or dedicated transmuters such as accelerator-driven systems (ADS) wherein fuels or targets containing long-lived nuclides are used.
To develop the fuels for MA transmutation, it is necessary to evaluate their behavior based on the thermochemical and mechanical properties of MA compounds. Our studies on MA compounds had been limited to those on Np and Am because of the difficulty of preparing Cm samples at mg scale with the high purity necessary for the abovementioned studies and of handling such samples. The most widely available isotope 244Cm is highly radioactive and decays to plutonium-240 (240Pu) almost immediately because the half-life of 244Cm is 18.1 years.
We developed a method for preparing high-purity Cm samples from aged 244Cm oxide samples. Removal of Pu and Am is necessary to obtain high-purity Cm samples from the starting material, a typical composition of which is 20% 244Cm-80% 240Pu oxide and which contains 243Am impurities (about 1%) (Fig.4-12). This method comprises four steps: dissolving the starting material in a mixture of nitric acid and H2O2 (oxidizing agent) with heating; removal of Pu by an anion exchange method; removal of Am by chromatographic separation using tertiary pyridine resin and a nitric acid/methanol mixed solution (Fig.4-13); and precipitation of Cm oxalate from the high-purity Cm solution (Fig.4-14).
We have prepared the oxide and nitride samples using the obtained high-purity Cm oxalates and have successfully measured their thermochemical properties. The method for preparing high-purity Cm samples, which will be used for the evaluation of MA transmutation fuel behavior, was established.
This study contains the results of “Basic actinide chemistry and physics research in close cooperation with hot laboratories” conducted under the Strategic Promotion Program for Basic Nuclear Research by the Ministry of Education, Culture, Sports, Science and Technology of Japan (MEXT).