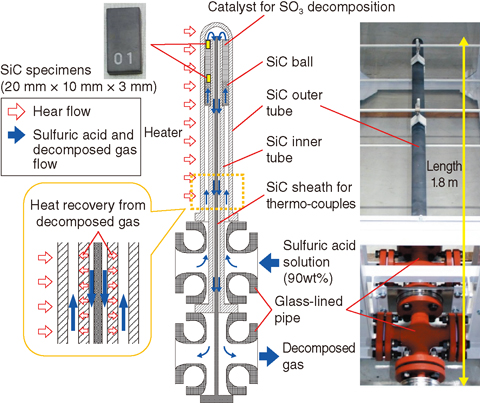
Fig.6-6 Overview of sulfuric acid decomposer made of SiC ceramics

Fig.6-7 Hydrogen-production test facility with total process components
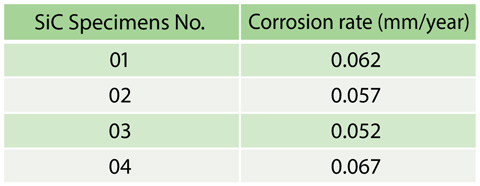
Table 6-1 Corrosion rates of SiC ceramics in a sulfuric acid decomposition reaction environment
A thermochemical iodine-sulfur process (IS process) is considered to be a promising hydrogen-production technology using the thermal energy produced by high-temperature gas-cooled reactors. The process splits water into hydrogen and oxygen by combining the chemical reactions of iodine and sulfur. Since it contains aggressive chemicals like sulfuric acid, one of the key tasks for realizing the process is the development of chemical reactor components made of industrial structural materials with heat- and corrosion-resistance abilities. We developed such a chemical reactor made of silicon carbide (SiC) ceramics to serve in a very harsh environment of sulfuric acid decomposition that no metallic material can resist.
SiC is known as a superior corrosion-resistant material, even under a sulfuric acid boiling condition around 300 ℃. However, information is limited concerning the corrosion-resistance behavior under higher-temperature sulfuric acid decomposition conditions around 850 ℃. SiC generally shows good corrosion resistance due to a stable oxide layer formed on the surface, but it is corroded by high-temperature steam. The subject environment of a sulfuric acid decomposition reaction is more complicated, as it not only has high temperature but also has a complicated corrosive environment including oxygen, steam, sulfur dioxide (SO2), and sulfur trioxide (SO3).
We adapted SiC ceramics to fabricate a sulfuric acid decomposer and evaluated the corrosion resistance of the SiC material under the reaction condition. As shown in Fig.6-6, the structure of the decomposer is simple; its design is achieved by coaxially assembling three SiC tubes and setting them on a manifold of glass-lined pipes. Sulfuric acid evaporates and decomposes into water, oxygen, and SO2 in the top region of the catalyst bed. This structure makes it possible to perform heat recovery from high-temperature decomposed gas flowing in an inner flow channel to the low-temperature sulfuric acid flowing in an outer channel. Moreover, no sealing area is provided in the high-temperature region.
We evaluated SiC corrosion resistance in the sulfuric acid decomposition environment. SiC specimens were placed in the SO3 decomposition catalyst bed. An exposure test of SiC to the environment was conducted for about 100 h to determine the corrosion rates from the weight changes of the specimens. No significant corrosion was observed from change in surface appearances. The determined corrosion rates were 0.05-0.07 mm/year, which are classified as perfect corrosion resistance, less than 0.05 mm/year, as shown in Table 6-1.
We then constructed a hydrogen production test facility that applies this type of decomposer, and all other plant components are made of industrial structural materials (Fig.6-7). Tests using the facility were launched to verify components’ integrity and to demonstrate the ability to produce hydrogen continuously.