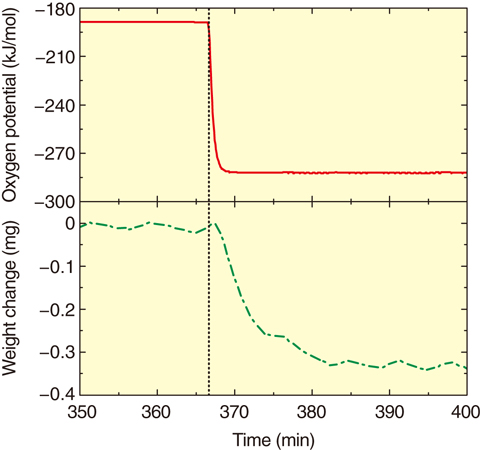
Fig.7-9 The change of oxygen potential and specimen weight
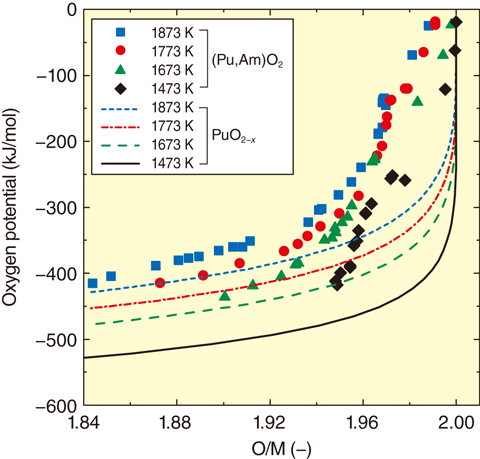
Fig.7-10 Oxygen potentials of (Pu0.928Am0.072)O2-x and PuO2-x
The spent nuclear fuels discharged from nuclear power plants contain minor actinides (MAs) such as americium (Am) and neptunium (Np). Since these MAs have high and long-term radiotoxicity, it is better to transmute MAs into stable or less-radiotoxic nuclides by leveraging fast reactors (FRs). MA-bearing oxide fuel is a major fuel candidate for FRs and is therefore required to measure various physical properties of the oxide fuel. Among MAs, Am significantly influences oxygen potential (ΔGO2), which is an important physical property. ΔGO2 has a direct effect upon the oxidation or reduction of oxide fuel. The change in ΔGO2 causes a change in the oxygen-to-metal (U, Pu, MAs) ratio (O/M ratio) in oxide fuel. It is well known that such a change significantly affects the sintering and irradiation behavior of oxide fuels.
In this study, we evaluate the relationship between ΔGO2 and the O/M ratio in Am-bearing PuO2 ((Pu,Am)O2) using thermogravimetry and estimate the effect of Am content upon ΔGO2. The oxygen is released into the atmosphere from a specimen or absorbed into the specimen from the atmosphere as the ΔGO2 value of the atmosphere changes. Weight change by release or absorption was measured using thermogravimetry (Fig.7-9). By means of measuring the change in the specimen weight under various atmospheres and temperatures, the relationship between ΔGO2 and the O/M ratio was evaluated.
The measured ΔGO2 data are plotted against the O/M ratio, together with the literature data for PuO2-x (Fig.7-10). ΔGO2 increased along with the increase in temperature. In particular, the ΔGO2 value of (Pu0.928Am0.072)O2-x markedly increased in the near-stoichiometriy (O/M = 2.00) region compared with that in PuO2 at the same temperature. Am is known to be more reductive than Pu; therefore, the increase in ΔGO2 of (Pu,Am)O2 in the near-stoichiometry region is considered to be caused by preferential reduction of Am.
We are going to conduct a test using Am-bearing MOX fuel to clarify the detailed mechanism by which Am affects ΔGO2.