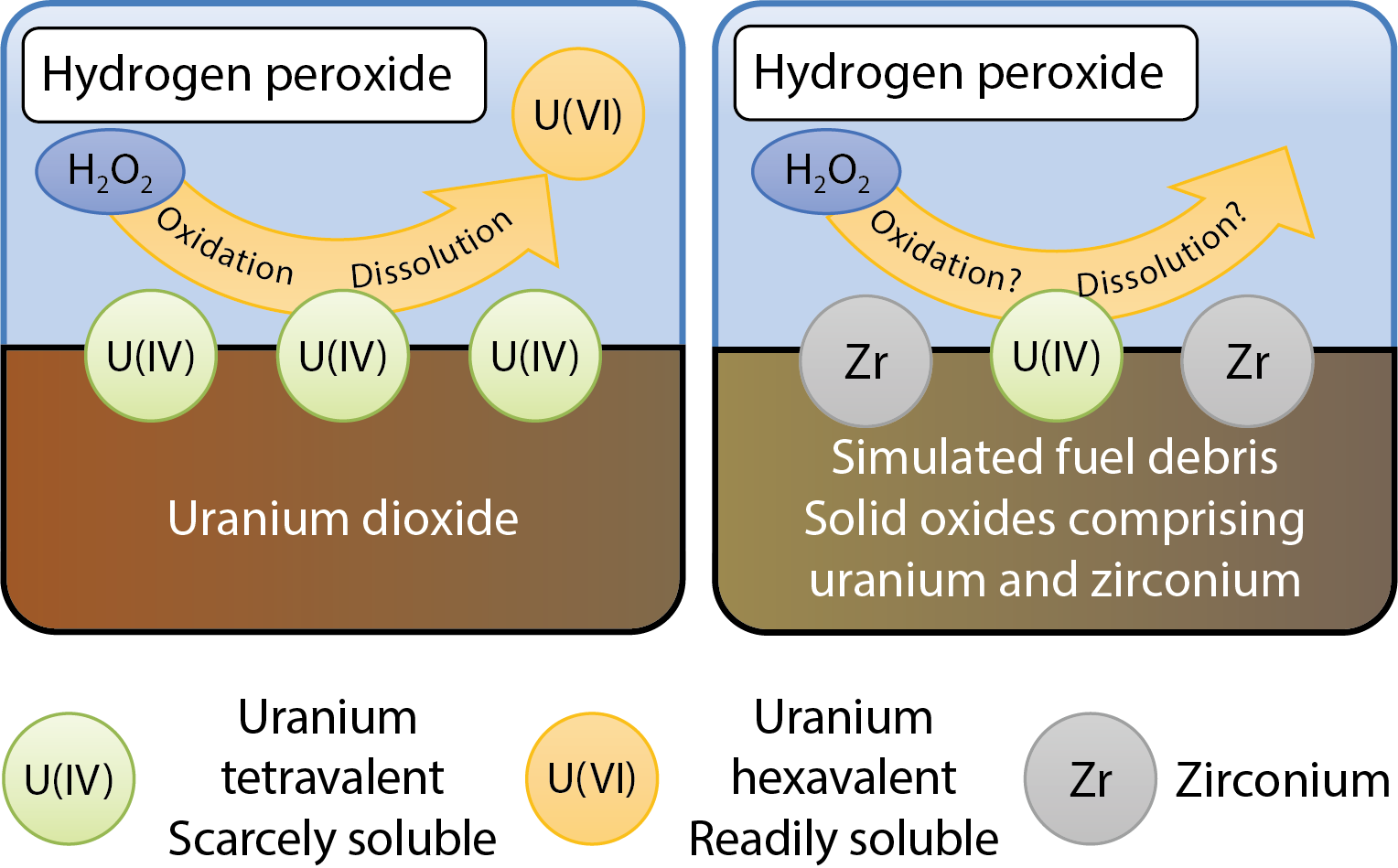
Fig.1-8 Reaction scheme of U dissolution by H2O2
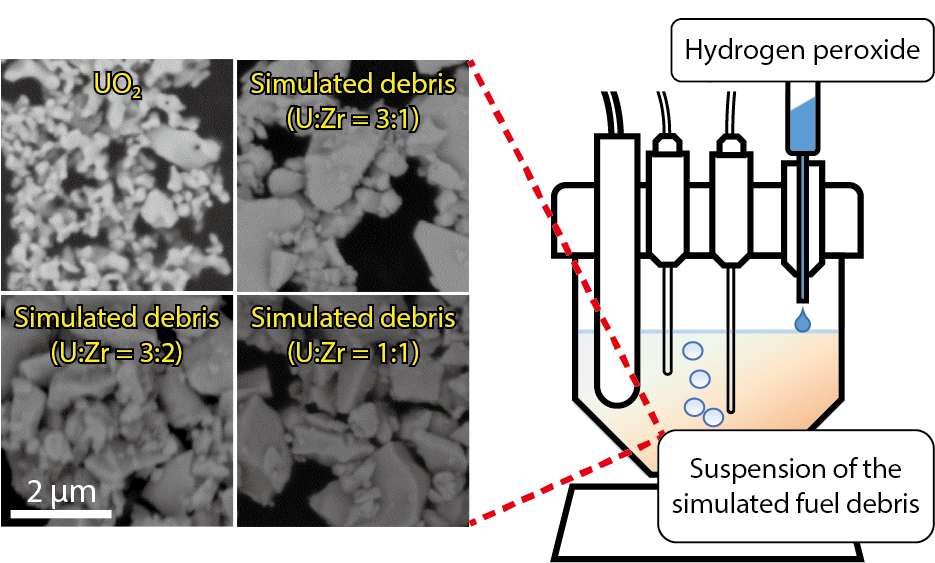
Fig.1-9 Scanning-electron-microscope (SEM) images of the U oxide samples and a schematic illustration of the setup for the H2O2 reaction experiment
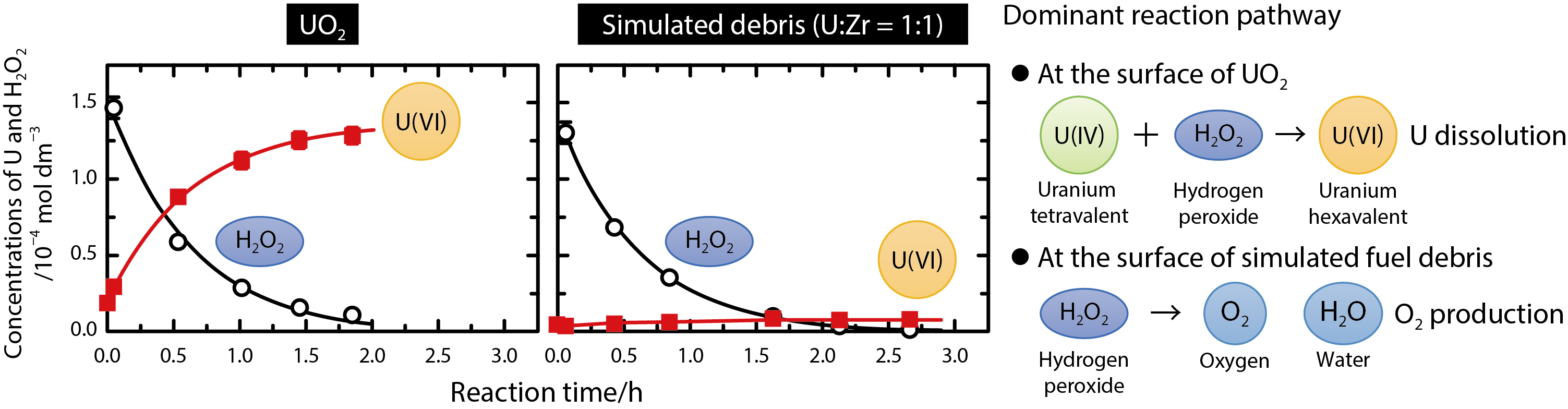
Fig.1-10 Comparison of the H2O2 reaction kinetics between UO2 and the simulated fuel debris
We have shown that uranium dioxide (UO2) becomes chemically stable by forming solid solution containing zirconium (Zr). The stabilization by Zr indicates that the molten fuel debris in the TEPCO’s Fukushima Dai-ichi NPS (1F) would be stable against dissolution by chemical reactions and would remain in the reactors until it is retrieved.
Our research on the chemical reaction of the molten fuel debris is based on an understanding of the chemical degradation of spent nuclear fuel under the conditions of deep geological disposal. The spent fuel emits intense ionizing radiation and produces hydrogen peroxide (H2O2) by decomposing water molecules. In the case of direct contact of the fuel with water, H2O2 consequently reacts with the fuel, inducing dissolution of uranium (U). The U dissolution is induced by oxidation of U at the surface of the fuel (Fig.1-8). In the UO2 matrix of the fuel, U is mainly in tetravalent state that is scarcely soluble in water. However, if tetravalent U is oxidized into hexavalent state, its solubility drastically increases. This sequence of chemical reactions is expected to occur for the fuel debris in 1F. However, little is known about the chemical behavior of the fuel debris in water, because the composition of fuel debris is far different from that of the usual UO2 fuel due to melting with the materials in the reactor core.
We have performed experiments regarding the dissolution of U by H2O2, and shown that it is significantly inhibited by the incorporation of Zr (Fig.1-9). U oxide containing Zr was used as simulated fuel debris, because the fuel debris is expected to contain Zr by melting with the fuel-cladding material. When the simulated debris was exposed to H2O2, the reaction of H2O2 proceeded at a rate comparable with that of UO2. However the dissolution of U was significantly inhibited (Fig.1-10). For example, the U dissolution from the simulated debris containing 50 % Zr in atomic ratio was only 4 % of that from UO2. This inhibited dissolution resulted from the effect of Zr promoting H2O2 decomposition on the surface. This reaction mechanism was confirmed by analysis of gaseous products of the reaction. The reaction of H2O2 with the simulated debris produced nearly the stoichiometric amount of oxygen (O2).
We are going to further investigate the basic chemistry of molten fuel in order to support technological development for the safe retrieval and management of the fuel debris in 1F.