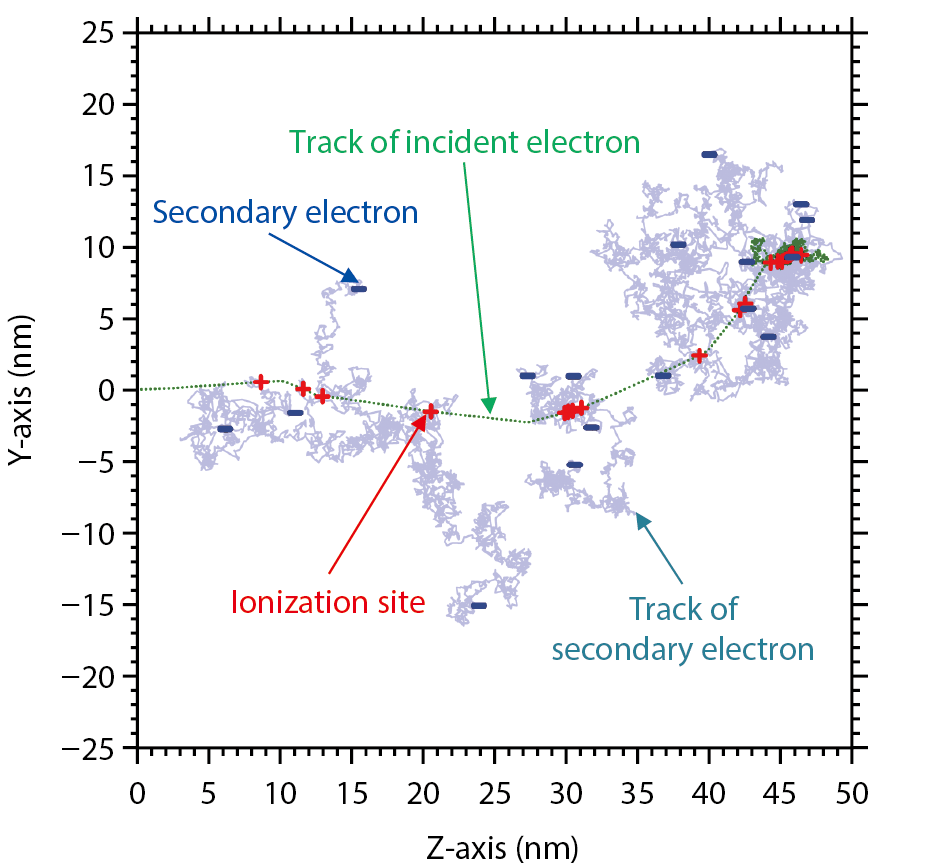
Fig.4-10 Electron tracks produced by an incident electron of 1 keV in water
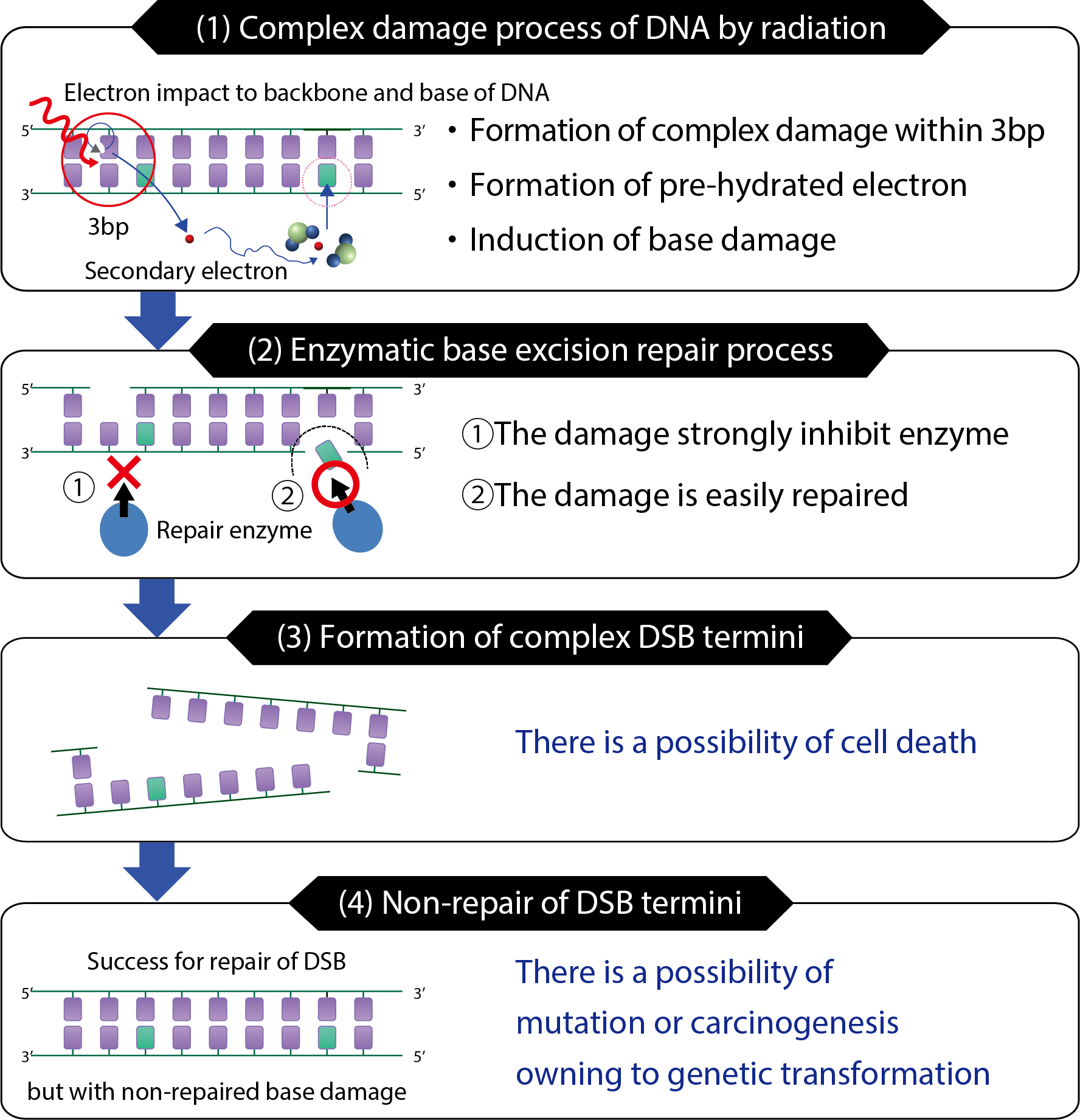
Fig.4-11 Relationship of DSB to base damage and biological effects
When DNA in a living cell is exposed to ionizing radiation, single- or double-strand breaks (SSBs or DSBs) or base damage of the DNA may occur. Although most DNA damage can be removed, it has been found experimentally that the repair efficiency was extremely low for simultaneous damage within 1 nm (called “clustered damage”). Clustered damage has been implicated in inducing harmful biological effects. However, such damage is hard to detect using experimental techniques.
In this study, a dynamic Monte Carlo code is developed to simulate electron irradiation to DNA, taking into account the Coulombic force between ion pairs. Collisional interactions between electrons and the DNA sample were analyzed using the code. Fig.4-10 shows the ionization inductions (+ points) and secondary-electron positions (− points) arising from the incidence of an electron with an energy of 1 keV upon water including DNA. First, it was found that the yield of the clustered damage constructed by the SSBs and base damage within 1 nm was constantly induced. It was also found that the secondary electrons deaccelerated below 1 eV were finally distributed over a few nm from the ionization sites. The analyses also clarified that reductive damage was produced by electron attachment to DNA when the electrons were located near such molecules.
Based on these analyses, we predicted that complex DNA damage sites were composed of clustered damage produced by the electron incident upon the DNA, and that the isolated base-damage site involved extremely low-energy electrons (Fig.4-11(1)). The isolated damage sites could be converted to SSBs by the elimination capability of the repair enzyme (Fig.4-11(2)). The DSBs with base damage in their termini were consequently formed if additional SSBs were produced in the complementary strand (Fig.4-11(3)). In this case, biological effects may be induced by the remaining base damage, even if the DSB could be repaired (Fig.4-11(4)).
This analysis indicated that extremely low-energy secondary electrons were involved in genetic transformation. This is a significant insight into the initial factors behind mutation and cancer inductions.
This work was supported by the Japan Society for the Promotion of Science (JSPS) KAKENHI Grants-in-Aid for Young Scientists (B) (No.15H02823), (No.16H02959), and Grant-in-Aid for Scientific Research (C) (No.17K07022).