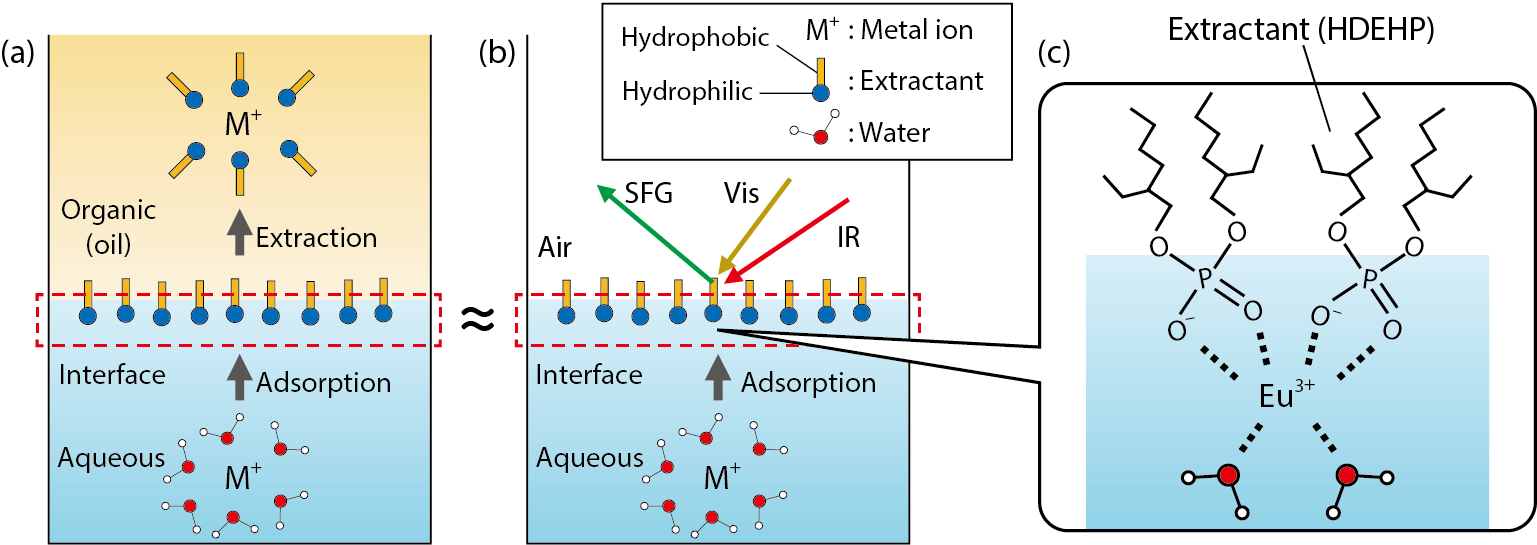
Fig.4-8 (a) Schematic of solvent extraction; (b) Diagram of the present research method; (c) Structure of a metal ion (Eu3+) at the interface clarified in this study
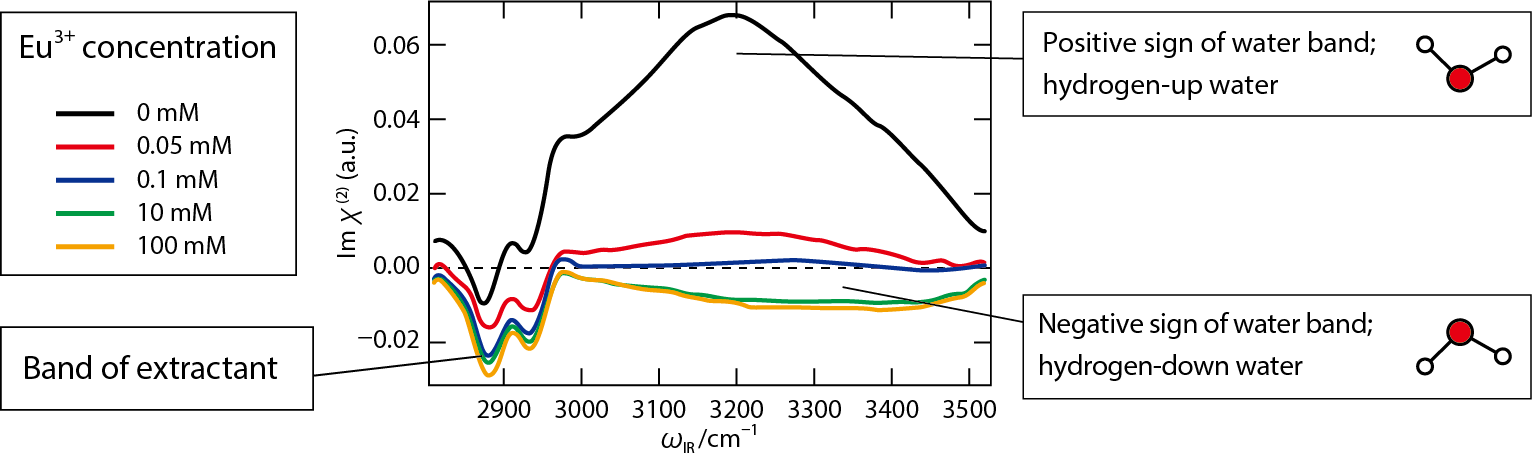
Fig.4-9 Vibrational spectra of the interface obtained by advanced laser spectroscopy: heterodyne-detected vibrational sum frequency generation spectroscopy
Solvent extraction is a method for separation of metals. In solvent extraction, an aqueous phase dissolving metal ions and an organic phase dissolving a chemical called the extractant are brought into contact with each other, and metal ions are transferred from the aqueous phase to the organic phase (Fig.4-8(a)). Solvent extraction is one of the most important methods of treating radioactive waste generated by nuclear-power generation. Improving solvent-extraction technology is an important research topic.
In order to improve solvent extraction technology, fundamental research has conventionally focused upon the structure of metal ions in the aqueous and organic phases. On the other hand, the phase transfer mechanism of metals from the aqueous phase to the organic phase through the organic/aqueous interface remains poorly understood. Therefore, if the phase transfer mechanism occurring at the interface can be revealed, it is expected that new clues will be obtained for improvement of solvent extraction technology. However, it is generally difficult to observe metal ions at the interface.
In order to observe metal ions just before transfer from the interface to the organic phase, metal ions adsorbed at the interface between air and water (surface of the aqueous solution) were observed by advanced laser spectroscopy (Fig.4-8(b)). In the present study, extraction of europium ions (Eu3+) using di-2-ethylhexyl phosphate extractant (HDEHP) was selected as a representative example of common solvent extractions, and the interface vibrational spectra of the system were obtained (Fig.4-9). As the concentration of Eu3+ increases, the positive sign of the water signal becomes negative. This indicates that interfacial water molecules pointing upward (hydrogen-up) change to a downward (hydrogen-down) orientation as Eu3+ is adsorbed to the interface. That is, interfacial water molecules are bonded to Eu3+ with hydrogen-down orientation (Fig.4-8(c)). The structure of Eu3+ sandwiched between HDEHP and water molecules has not been reported so far in the organic or aqueous phases, so that the Eu3+ structure at the interface is unique.
Based on the findings mentioned above, we proposed a reaction model for solvent extraction of Eu3+ using HDEHP as follows. In the aqueous phase, Eu3+ is surrounded by water molecules, but Eu3+ is sandwiched between HDEHP and water molecules at the interface; subsequently, Eu3+ is extracted in the organic phase and surrounded by HDEHP.
In future work, we will conduct studies that lead to improvement of the extraction rate, separation efficiency, etc., by clarifying what is happening at the interface in actual solvent extractions of radioactive waste.
This work was supported by the Japan Society for the Promotion of Science (JSPS) KAKENHI Grant-in-Aid for Young Scientists (B) (No.17K14919).