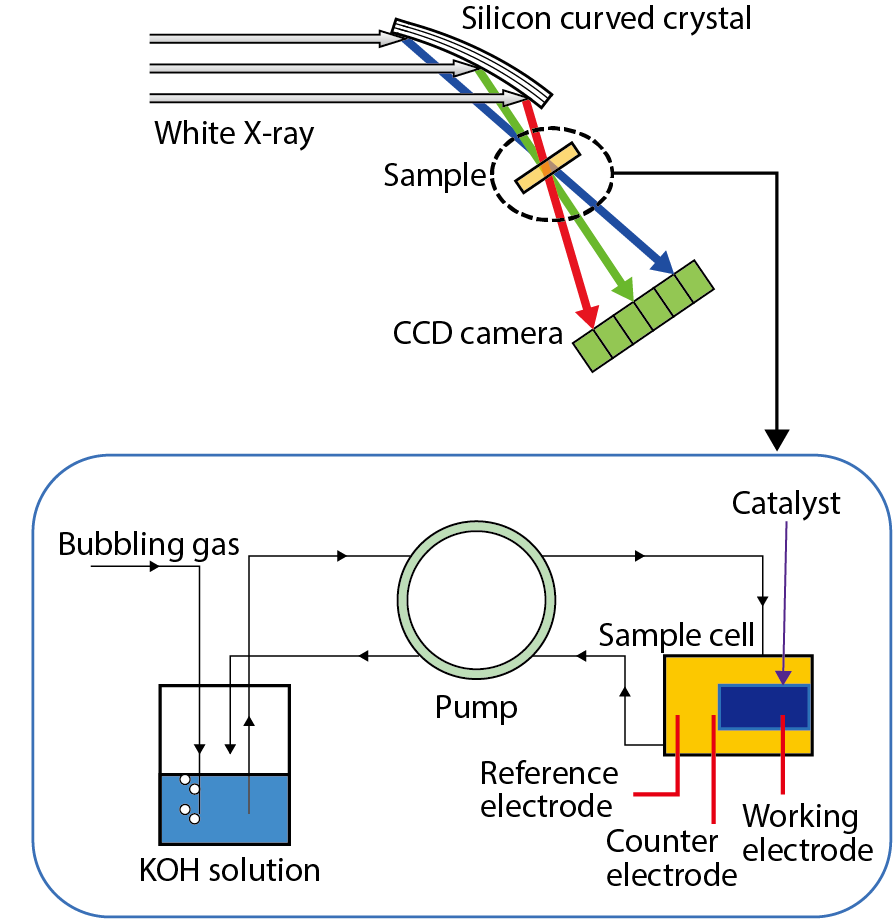
Fig.5-19 Schematic pictures of X-ray-absorption spectroscopy system
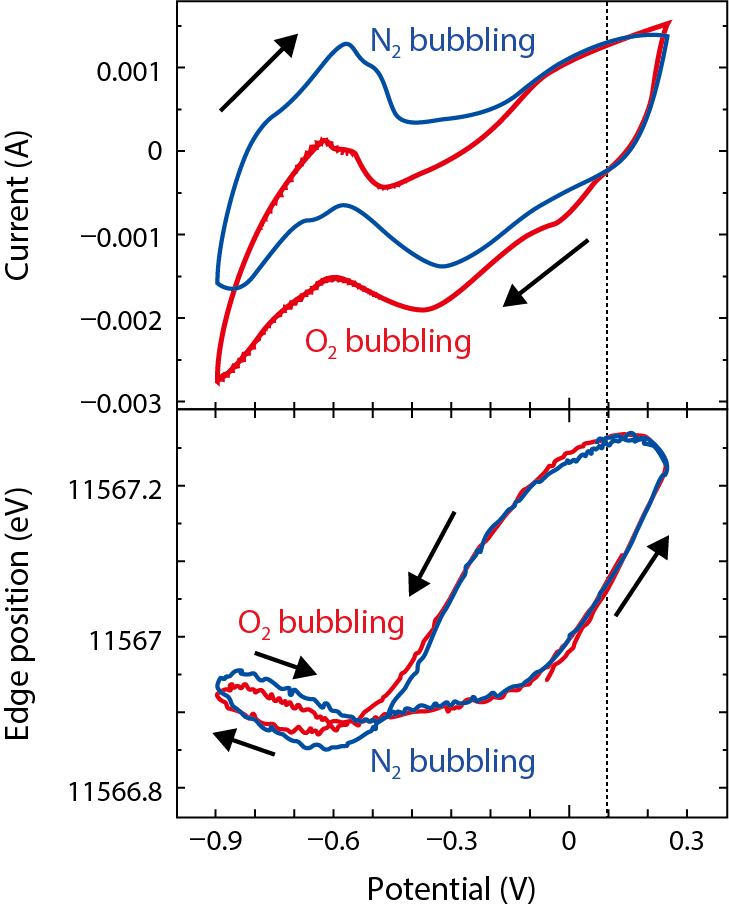
Fig.5-20 Simultaneous observation results of electric current and X-ray-absorption-edge position
Fuel-cell development has attracted a great deal of attention for a long time. Fuel cells have the advantage of not discharging any carbon dioxide at all during power generation; however, they also have some drawbacks, such as using precious platinum metals as electrocatalysts. Platinum is considered to be degraded by oxidation and it is important to investigate how platinum-metal catalysts are oxidized during fuel-cell operation.
X-ray-absorption spectroscopy is an element-selective technique, which means that only structural information about platinum can be obtained among all electrode components. The oxidation state is determined by the position of the X-ray-absorption edge. Fig.5-19 shows the outlines of the dispersive X-ray-absorption-spectroscopy-observation system, as well as the sample setting adopted in this study. While we have to change X-ray energy by moving the monochromator in the normal case, the displayed system can detect absorption spectra without any mechanical-motion process; this means that absorption spectra can be measured within a short time. We have utilized this system for high-speed observation of time-resolved X-ray-absorption spectroscopy with a sweeping electric potential in order to reveal the oxidation states of the platinum-metal catalyst during electrocatalytic reactions.
The oxygen-reduction reaction is considered to be a rate-limiting process for fuel-cell operation. In this experiment, oxygen bubbling was applied to the solution in which the electrode was immersed to realize the oxygen-reduction reaction, and compared with the result of nitrogen bubbling that purges oxygen gas. Fig.5-20 shows the electric current caused by the catalytic reaction and the absorption-edge-position of platinum determined by X-ray-absorption spectroscopy measured at the same time. As the potential is reduced from the high-potential side, the current with oxygen bubbling increases in the negative direction under 0.1 V. This is due to a new current caused by the oxygen-reduction reaction. At this reaction-onset potential, the position of the X-ray-absorption edge retains a relatively high value, indicating that the surface of the platinum catalyst is covered by the oxide layer when the oxygen-reduction reaction starts, because the higher the position of the absorption edge, the more that the platinum catalyst is oxidized. It was also found that the reduction reaction of the oxide layer of the platinum-catalyst surface was inhibited by the presence of oxygen gas. The absorption-edge positions decrease as the potential is lowered, but the oxygen-bubbling case shows a gentler slope.
This study has revealed the oxidation state of the platinum catalyst at the reaction-onset potential. We believe that these results will lead to the development of a high-performance catalyst that expands the oxygen-reduction reaction to the higher-potential side.
This work was supported by the Japan Society for the Promotion of Science (JSPS) KAKENHI Grant-in-Aid for Young Scientists (B) (No.16K17540).
<Previous: 5-6 | Next: 6 HTGR Hydrogen and Heat Application Research>