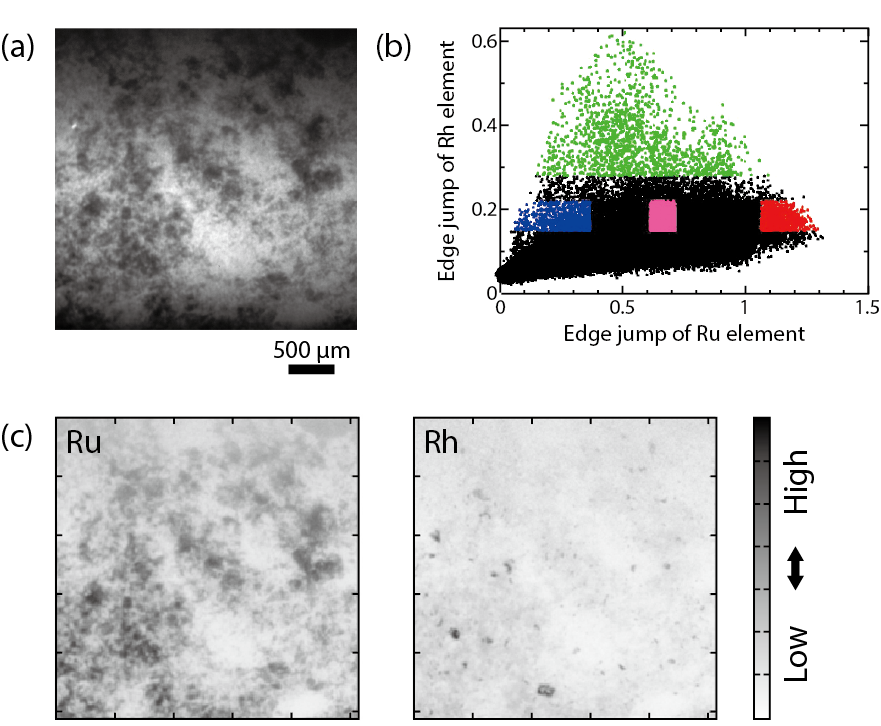
Fig.5-17 Distribution correlation between Ru and Rh elements in borosilicate glass
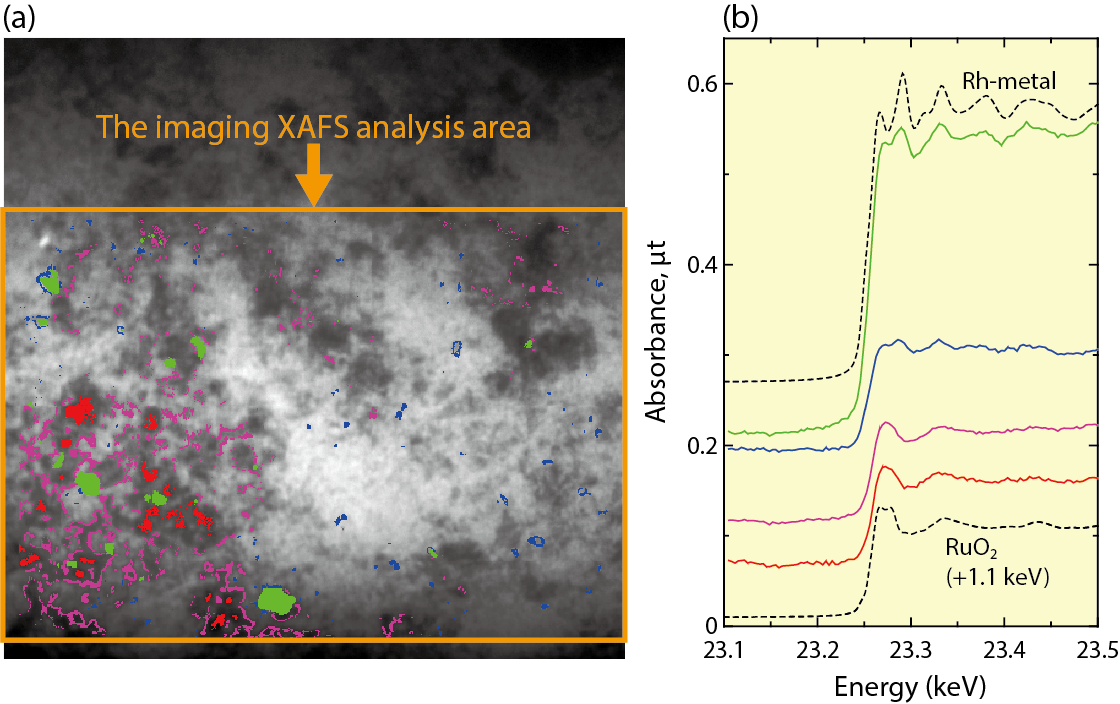
Fig.5-18 Imaging XAFS spectra derived based on the distribution correlation
The high-level liquid waste (HLLW) created by reprocessing spent nuclear fuel is mixed with molten borosilicate glass at high temperature inside a glass melter. Geological disposal of vitrified radioactive waste is planned after intermediate storage in Japan. Because there are more than 30 kinds of elements in the vitrified waste, some of their compounds are not contained stably in the borosilicate glass. It is very important to investigate the chemical state and coordination structure of these elements for safe management of vitrified waste. Synchrotron radiation-based X-ray absorption fine structure (XAFS) analysis, which offers high selectivity of specific elements and high sensitivity to diluted components, has been widely used in the chemical analysis of simulated vitrified waste. We have developed and used the imaging XAFS technique, which offers position sensitivity in addition to elemental selectivity. The distribution of each element and the XAFS signals corresponding to selected pixels on the transmission X-ray image are obtained through imaging XAFS analysis. It is known that platinum-group metals (PGM; Ru, Rh, and Pd) are aggregated in borosilicate glass because of their low solubility: they settle down and are deposited at the bottom of the glass melter. This is a typical problem in the vitrification process. Therefore, we study the behavior of PGM elements in borosilicate glass using the imaging XAFS technique in cooperation with the TRP Decommissioning Center.
Fig.5-17 shows imaging XAFS analysis results for borosilicate glass containing simulated HLLW components. It is clear from the two mapping figures that the distributions of Ru and Rh elements are not uniform but are rather aggregated in the glass sample. This behavior is already documented and need not be investigated using a synchrotron facility. The additional remarkable feature of this study is that the imaging XAFS spectrum was obtained separately based on correlation information of the Ru and Rh distributions. The chemical state of Rh in borosilicate glass is usually a mixture of Rh metal and its oxides. It has been reported that the only Rh oxide detected was RhO2, while the Rh2O3 that was expected thermodynamically was not detected in the borosilicate glass.
The X–Y plot of the absorption edge jump values for Ru and Rh, as obtained he from the XAFS spectra for each pixel on the transmission X-ray image, are shown in Fig.5-17(b). We selected three areas in the figure: (1) the red area, where a lot of Ru is distributed; (2) the blue area, where little Ru is distributed; and (3) the green area, where a lot of Rh is distributed. Since each point of the X–Y plot and pixel on the image form a pair, we can identify pixels corresponding to the selected area shown in Fig.5-18(a). The imaging XAFS spectra obtained from the X-ray intensities of the three selected areas (red, blue, and green) are shown in Fig.5-18(b). Imaging XAFS analyses showed that the chemical form of Rh was mainly RhO2, where the concentration of Ru was very high (red area) and metallic when the Ru concentration was low (blue area). In the very highly concentrated Rh area, its chemical form was metallic.
These results suggest that the chemical form of Rh is basically metallic in the borosilicate glass but becomes RhO2 where its distribution accords with that of Ru. The chemical state of Rh in the vitrified waste is more strongly controlled by the distribution correlation with Ru elements, rather than the thermodynamic properties of Rh compounds. We will use the imaging XAFS technique to clarify the complicated chemical behavior in the vitrified waste and contribute to the advancement of vitrification technology.
This work was supported by the Japan Society for the Promotion of Science (JSPS) KAKENHI Grant-in-Aid for Scientific Research (C) (No.15K04739).