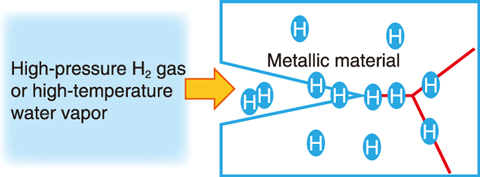
Fig.9-8 Conceptual diagram of hydrogen embrittlement of metallic materials
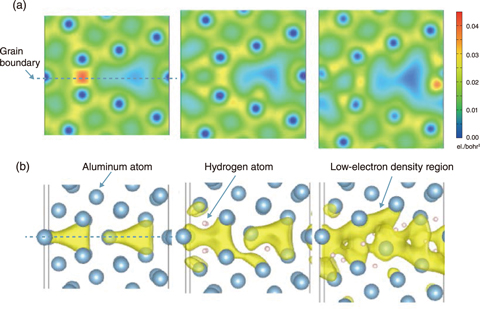
Fig.9-9 Separation of aluminum grain boundaries by penetration of hydrogen atoms
As the materials inside a light water reactor are exposed to strong radiation and high-temperature water vapor, they must be resilient against deterioration over a long period. In some next-generation nuclear reactors, liquid metal is used as a refrigerant instead of water; either way, material deterioration due to liquid metal or hydrogen and oxygen must be addressed and prevented. The Center for Computational Science and e-Systems has aimed to clarify the mechanism of material degradation caused by the invasion of substances in the environment using simulations (Fig.9-8). As an application development, the degradation mechanism of aluminum by hydrogen was investigated. Hydrogen has been considered a promising energy source in the fight against global warming, but usage requires materials that can safely store hydrogen. However, hydrogen weakens many metals in a phenomenon known as hydrogen embrittlement. The aluminum alloy currently used for hydrogen storage is known to be less susceptible to hydrogen embrittlement under normal conditions, but it is known to be susceptible to embrittlement under more severe conditions, and its mechanism has not been clarified. The mechanism of hydrogen embrittlement must be clarified to allow for the design of better materials.
The effect of hydrogen atoms on aluminum was therefore investigated using a first-principle calculation method to solve the Schrodinger equation and thus better understand the behavior of the electrons responsible for the properties of matter. In particular, calculations on the grain boundaries where hydrogen atoms to penetrate revealed interesting phenomena. In the case of iron and other materials, when hydrogen atoms enter a grain boundary, they expand up to a certain extent. However, when a hydrogen atom enters the crystal grain boundary in aluminum, the aluminum tends to expand endlessly and separate spontaneously to allow the hydrogen atom to enter, as shown in Fig.9-9.
Aluminum has a lower bonding energy between atoms than iron. A mechanism was discovered in which hydrogen atoms enter the gap and bond with aluminum atoms while breaking the bonds between aluminum atoms, thus stabilizing the structure. Furthermore, hydrogen atoms were shown to penetrate and split the material by pulling slowly even with a weak force. Such phenomena only occurred in environments with higher pressures and corrosion. In this way, the research methods developed in the research of nuclear materials are also effective in the research of metal materials for industrial use, producing ripple effects.
This work was partly supported by the Japan Science and Technology Agency (JST) (No.JPMJSK1412).