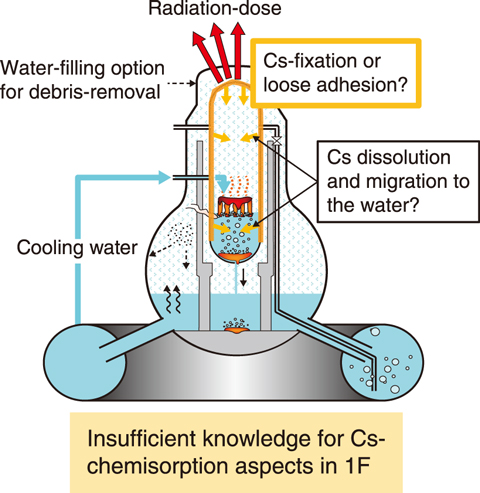
Fig.1-12 Cesium (Cs) transport during SA
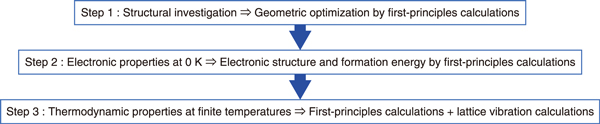
Fig.1-13 Calculation of thermodynamic properties by first-principles calculations and lattice vibration calculations
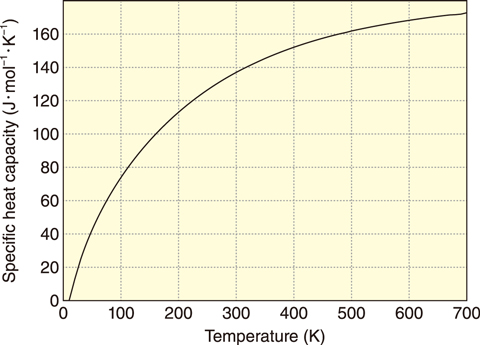
Fig.1-14 Calculated specific heat capacity of CsFeSiO4
In a severe accident (SA) such as the TEPCO’s Fukushima Daiichi NPS (1F) accident, fission products (FPs) are released into the reactor buildings and environments due to the melting of the core and damage to the reactor vessels. To respond to the accident and to formulate evacuation plans and post-accident decommission processes, it is necessary to understand the release and transport behaviors of FPs and to evaluate the exposure of the public and workers accurately. The release and transport behaviors of FPs depend on the chemical behaviors such as chemical reactions and properties. As shown in Fig.1-12, cesium (Cs) causes chemical reactions with structural materials inside the reactor at high temperatures during an SA; Cs fixed by chemisorption becomes a major radiation source in the reactor. To clarify the behavior of chemisorbed Cs, it is necessary to investigate the chemical species formed during chemisorption.
To investigate these chemical species, a series of Cs chemisorption experiments were performed on stainless steel (SS), which is used as a structural material. The results confirmed that compounds such as CsFeSiO4, CsFeO2, and Cs2Si4O9 were formed by reacting with iron (Fe) and silicon (Si) contained in SS during chemisorption. Chemical equilibrium calculations were performed using the thermodynamic data of these compounds to predict the chemical species formed during SAs. However, the thermodynamic data of CsFeSiO4 required for this prediction have not been reported.
A method to predict thermodynamic properties without measured data was therefore developed based on a combination of first-principles and lattice vibration calculations. This calculation process, summarized in Fig.1-13, involves structural investigations, the calculation of electronic properties at 0 K by first-principles calculations, and the determination of thermodynamic properties at finite temperatures by lattice vibration calculations performed based on these investigations. The validity of this approach was then verified by comparison with reported thermodynamic data such as the standard enthalpy of formation at 298 K, the standard entropy at 298 K, and the temperature dependence of the specific heat capacity for Cs2Si2O5 and Cs2Si4O9. Applications of this approach, furthermore, made it possible to predict the thermodynamic data of CsFeSiO4. As an example, the calculated specific heat capacity of CsFeSiO4 is shown in Fig.1-14.
The developed calculation approach proposed here allowed for predicting Cs chemical species chemisorbed on structural materials. Future work will aim to improve the proposed method and include a more in-depth verification of the proposed method with experimental data. Additionally, the proposed method can be used to contribute to assessments on exposures in the 1F reactor.
(Chikashi Suzuki)