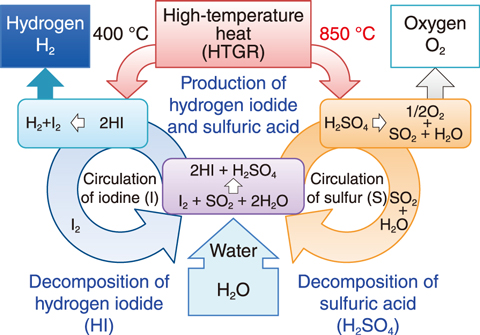
Fig.6-9 Schematic of IS process for the thermochemical production of hydrogen
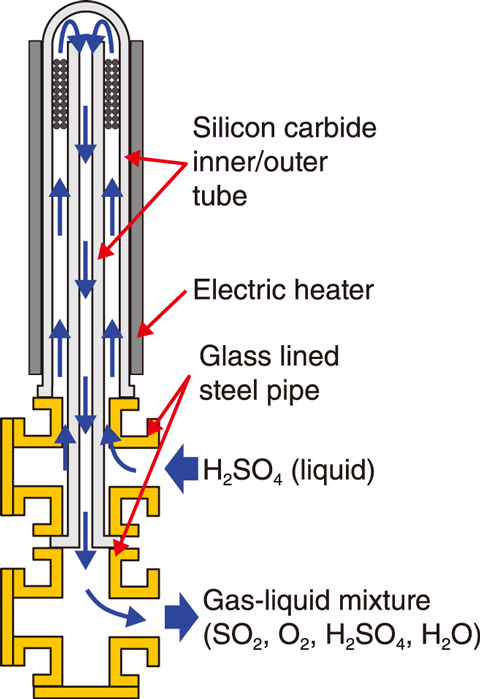
Fig.6-10 Sulfuric acid decomposer made of silicon carbide (SiC)
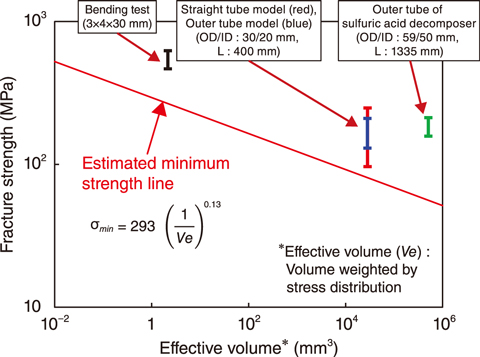
Fig.6-11 Estimated minimum strength equation and experimental validation using SiC structure
Hydrogen and oxygen can be produced through a chemical reaction between iodine (I) and sulfur (S) in the thermochemical water-splitting iodine-sulfur (IS) process, as shown in Fig.6-9. As the IS process uses highly corrosive chemicals such as hydrogen iodide and sulfuric acid at high temperatures, the materials used for the process must be corrosion resistant and heat resistant.
During the IS process, the sulfuric acid decomposer decomposes extremely corrosive sulfuric acid at temperatures up to 850 ℃. Due to its corrosion and heat resistance, existing metal materials cannot be used for the decomposer. A sulfuric acid decomposer made of silicon carbide (SiC), which is a ceramic material with excellent heat and corrosion resistance, was thus proposed for the sulfuric acid boiling/evaporation part of the decomposer, as shown in Fig.6-10; the performance and reliability of this proposed decomposer have been confirmed by continuous hydrogen production tests.
Because the heat source of a commercial IS plant is high-pressure helium gas from an HTGR, the SiC sulfuric acid decomposer must be approved by the High Pressure Gas Safety Act. However, because the average strength of ceramic materials decreases with increasing volume, it is not possible to evaluate the strength of a full-scale structure from the strength of a small bend test specimen according to the JIS standard.
A strength evaluation method that can be applied to a full-size ceramic apparatus was therefore developed. The objective of the strength evaluation method was to determine the minimum strength of the structure so that it can be designed considering the strength variation of ceramic materials. The minimum strength estimation equation was developed by combining the relationship between crack size and fracture strength (fracture mechanics) and the relationship between crack distribution and fracture strength (fracture probability); the resulting minimum strength equation is shown in Fig.6-11. Destructive testing of SiC specimens of various sizes up to the actual size of the decomposer was then performed by applying water pressure to obtain the strength distribution equation and validate the calculated minimum strength equation.
The results, as shown in Fig.6-11, demonstrated that all of the fracture strengths were above the minimum strength derived from the estimation equation. Thus, it was confirmed that the developed strength estimation equation could be used to evaluate the minimum strength for full-scale SiC structures. This result paves the way for the evaluation of the strength of the SiC structure in the sulfuric acid decomposer.
Future work will include establishing the design method of the SiC structure by adding a safety factor and enhancing is reliability of through the use of newly developed corrosion-resistant metals.
(Hiroaki Takegami)