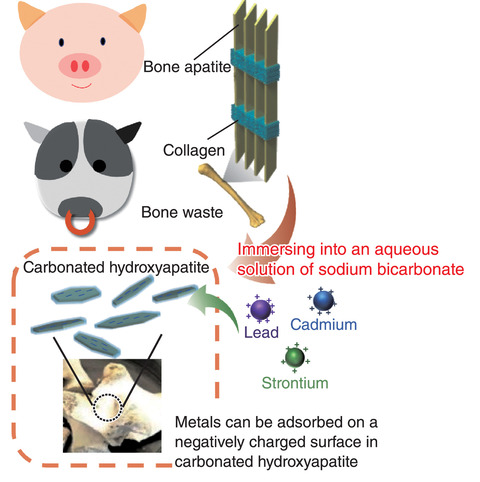
Fig.5-10 Schematic of metal adsorbents using bone waste
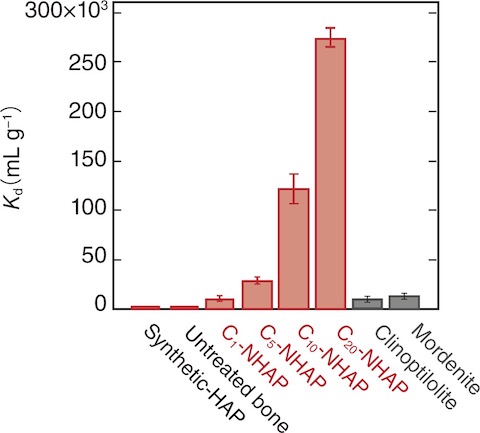
Fig.5-11 Distribution coefficients (Kd) of Cx-NHAPs, natural bone, synthesized HAPs, clinoptilolite, and mordenite
Decontamination of a wide range of pollutants has been identified as a key challenge for building a sustainable and safe society, especially after the accident at TEPCO's Fukushima Daiichi NPS in 2011. The development of effective methods for removing pollutants from the environment remains an important research topic.
Biological hydroxyapatite (HAP) that can be extracted from animal bone is an interesting material because it exhibits greater adsorptivity for cadmium (Cd), strontium (Sr), and uranium (U) than those exhibited by synthetic HAPs. However, the ion-adsorption performance is lower than that of other conventional materials. The ion adsorptivity of biological HAP has been considered to depend on its highly substituted carbonate ions, but the adsorption mechanisms have not yet been fully elucidated.
This study aims to use bone waste to develop low-cost effective metal adsorbents that can adsorb 90Sr and heavy metals (Fig.5-10). In this study, we focused on the correlation between the high carbonation substitution ratio, which is the most characteristic property of biological HAP, and adsorption performance. First, bulk pig bone was immersed in water and autoclaved to eliminate the residual organic compounds. Then, the heat-treated bone was immersed in aqueous sodium bicarbonate (NaHCO3) solutions to obtain highly carbonated nanohydroxyapatites (C-NHAP). We investigated its performance with respect to the adsorption of Sr2- and Cd2-. The distribution coefficient (Kd) of the C-NHAP for Sr2- was approximately 20 and 250 times greater than those of clinoptilolite and the untreated bone, respectively (Fig.5-11). The Kd value of the C-NHAP for Cd2- was approximately 370 and 23 times greater than those of the untreated bone and clinoptilolite, respectively. The amount of introduced CO32- into C-NHAP increased with the increasing NaHCO3 concentration in the immersion solution. With the increasing amount of CO32- ions introduced into the C-NHAP, it exhibited a greater ion-adsorption performance.
The CO32- substitution approach presented in this study would be a useful strategy for the development of high-performance HAP adsorbents for toxic ions, especially 90Sr, by efficiently using waste bones.
(Yurina Sekine)