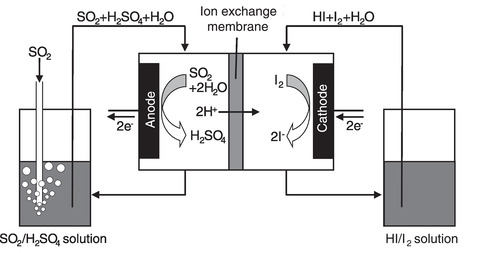
Fig.6-14 Schematic of the Bunsen reaction using the ion-exchange membrane
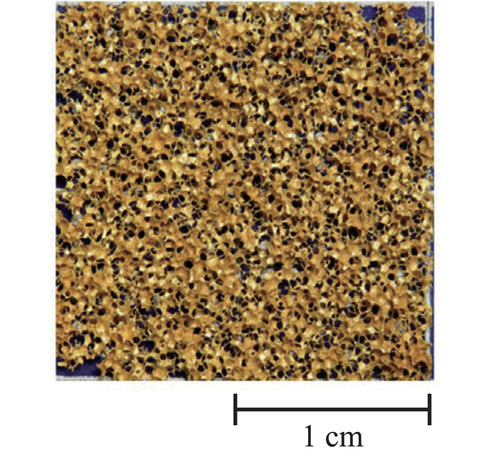
Fig.6-15 Microscopic outlook of the porous Au electrode
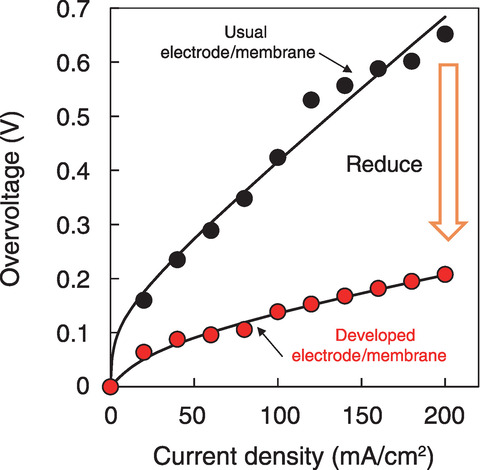
Fig.6-16 Current density dependence of the Bunsen reaction overvoltage
The iodine-sulfur (IS) water-splitting H2 production process as the heat application of high temperature gas-cooled reactors is investigated to realize the hydrogen (H2) economy. The IS process is composed of sulfur and iodine chemical reactions.
The Bunsen reaction, which is a starting reaction of the IS process, produces sulfuric and hydriodic acids by mixing iodine and sulfur dioxide with water. The membrane for the Bunsen reaction method is investigated to allow the Bunsen reaction to proceed using an electric cell equipped with an ion-exchange membrane (Fig.6-14). The conventional Bunsen reactor required the separation process of two acid solutions after a reaction. In contrast, no solution separation process is needed because the two acids can be separately produced in a cell partitioned into two channels by the membrane. However, in the membrane for the Bunsen reaction assembled with the usual electrodes and membrane, the overvoltage was excessively high for practical application to the IS process. The overvoltage originated from the anode reaction at the anode electrode and the membrane resistance caused by the proton permeation. Therefore, the novel electrode and the ion-exchange membrane were developed to reduce the overvoltage.
First, the porous Au electrode was developed in collaboration with Shibaura Institute of Technology (Fig.6-15). The porous electrode that enhanced the effective surface area allowed the anode reaction to easily proceed, indicating that the overvoltage was reduced.
Second, the ion-exchange membrane, which introduced many ion-exchange groups compared with the usual membrane to improve the conductivity, was prepared through the National Institutes for Quantum Science and Technology technique. The prepared membrane enabled the reduction of the membrane resistance.
Finally, the membrane for the Bunsen reaction was operated using the electric cell assembled with the developed electrode and membrane. The stoichiometric Bunsen reaction was confirmed to proceed. Moreover, the overvoltage was 0.21 V at 200 mA/cm2, which is 1/3 lower than that of the usual setup (Fig.6-16). In summary, the practical process efficiency for the technical applicability of the membrane for the Bunsen reaction can be achieved.
This work was supported by the Strategic Innovative Promotion (SIP) program of the Council for Science, Technology and Innovation (Cabinet Office, Government of Japan).
(Nobuyuki Tanaka)