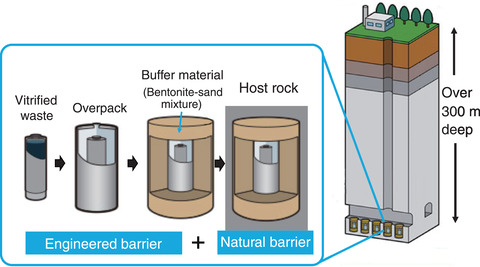
Fig.8-27 Basic concept of HLW disposal
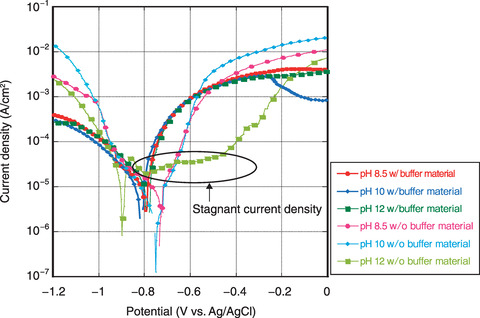
Fig.8-28 Anodic/cathodic polarization curves measured in 10% SSW with and without buffer material
Current designs for barriers for geological disposal sites of high-level radioactive waste (HLW) in Japan use carbon steel overpack containers surrounded by a bentonite-sand buffer material, as shown in Fig.8-27. Passivation is a process in which a corrosion-resistant thin oxide film (i.e., passivation film) is formed on the metal surface. If carbon steel is passivated, localized corrosion occurs; otherwise, general corrosion occurs. Under general corrosion, the containment life of the carbon steel overpack can be determined by the corrosion depth, which is based on the corrosion rate. On the other hand, localized corrosion can cause severe corrosion, such as pitting, which can reduce the lifespan of carbon steel overpacks. Therefore, to ensure that carbon steel overpacks that have not been passivated (and thus that do not incur localized corrosion), the conditions causing passivation to occur in the disposal environment must be understood. Researchers have thus investigated the conditions that lead to the passivation of carbon steel, such as groundwater environments rich in carbonic acid*1. Considering the transportation of HLW, coastal waste disposal locations have become preferred*2; thus, the corrosion behavior of steel given the groundwater environment of coastal areas should be studied.
Therefore, we studied the corrosion behavior of carbon steel with and without buffer material (bentonite) using a solution of diluted synthetic seawater (10% SSW) to mimic the groundwater in coastal areas, which comprises a mixture of water originating from seawater and freshwater. The solution pH was adjusted to 8.5, 10, and 12 to investigate the passivation behavior in a high pH environment, as carbon steel is known to passivate easily in high-pH environments. A low-oxygen atmosphere was used to simulate the underground environment. A carbon steel electrode was directly immersed in the solution or embedded in the buffer material before the solution was impregnated. The polarization measurements were performed in a low-oxygen glove box.
The resulting anodic/cathodic polarization curves are shown in Fig.8-28. Without the buffer material, current density stagnation with passivation was observed at a pH of 12; however, no passivation was observed in any condition when the buffer material was included. Even if the dilution ratio of synthetic seawater has been changed, localized corrosion was observed only at high pH without the buffer material and general corrosion was observed under the other conditions. The pH buffering function of the bentonite reduced the pH of the solution in contact with carbon steel (i.e., bentonite pore water), thereby preventing the passivation of the carbon steel even in high-pH solutions.
Overall, using a bentonite buffer prevented the passivation of carbon steel in coastal groundwater even in a high-pH environment. Our results suggest that the main corrosion mode of carbon steel surrounded by a buffer material in this environment is general corrosion. Additionally, we confirmed the applicability of the corrosion evaluation model based on general corrosion to the corrosion of carbon steel overpacks used in geological disposal in coastal areas.
This study was funded by the Ministry of Economy, Trade and Industry (METI) of Japan through the program “Advancement of the geological disposal system in coastal regions” (JFY2017-2018).
(Ayami Kitayama)
*1 Taniguchi, N. et al., The Assessment of Corrosion Type and Corrosion Rate of Carbon Steel in Compacted Bentonite, JNC-TN8400 99-003, 1999, 88p.
*2 Advisory Committee for Natural Resources and Energy, Examination Results of Requirements and Standards for Presentation of Regional Scientific Characteristics for Geological Disposal, 2017, p.59-62.
<Previous: 8-9 | Next: 9 Computational Science and E-Systems Research>