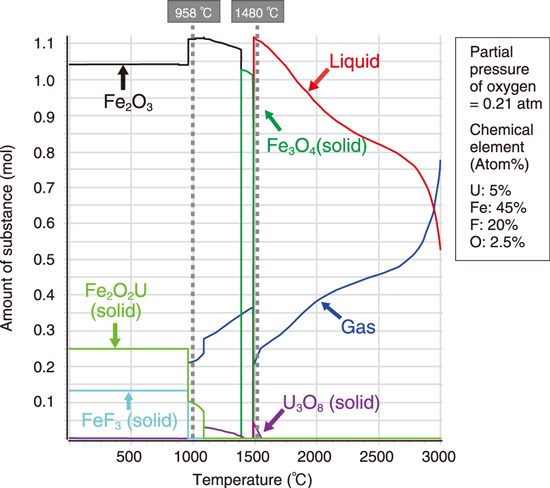
Fig.8-11 Thermodynamically analyzed phase stability of iron and uranium compounds in the atmosphere
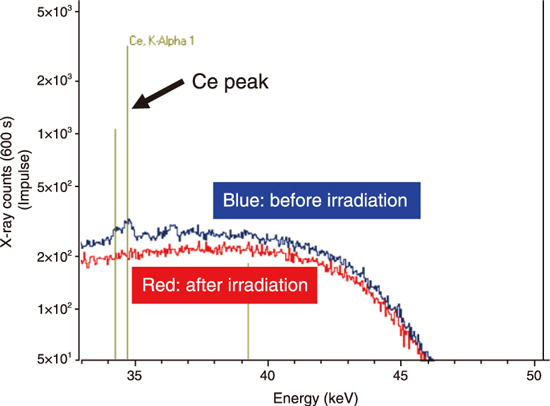
Fig.8-12 X-ray fluorescence spectra of cerium oxide applied to the painted steel surface before (blue) and after (red) irradiations
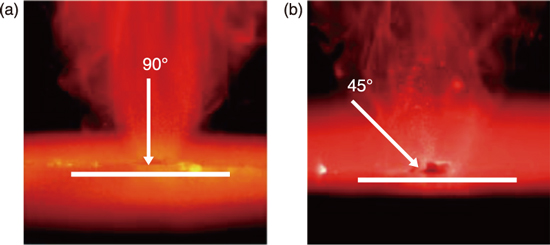
Fig.8-13 Airborne dust behavior under different irradiation angles
The dismantling of the Ningyo-toge Environmental Engineering Center generates approximately 130,000 tons of waste which contains approximately 80% nonradioactive wastes (NRs). Though some steels painted with a coating film can be disposed as NRs, there is the possibility that their painted surface are potentially contaminated. Rotary surface grinding has been conventionally used to remove such steel surface painted with a coating film, but it requires protective equipment and clothing to prevent dispersing and inhaling airborne dusts.
Laser cleaning is a promising surface removal technique, because it is an on-site surface removal technique, which allows the indirect operation keeping a safe distance from its object. Consequently, protective equipment and clothing can be less necessary, workload can be lightened in the controlled area, and secondary wastes can be reduced. However, reports of uranium and iron compounds behaviors at high temperatures under laser irradiation are limited, and it is indispensable to develop a technique to recover airborne dusts removed from the surface through laser cleaning.
In this work, we studied phase stability of uranium and iron compounds, separation behavior of powder-like uranium compounds, and scattered direction of removed airborne dusts under laser irradiation for the performance evaluation of laser cleaning applied to the uranium-contaminated steel surface painted with a coating film.
First, we thermodynamically focused the phase stability of uranium and iron compounds at high temperatures. This is because uranium compounds on the coating film could be dissolved into the base metal through the heating under laser irradiation. As shown in Fig.8-11, uranium forms gas phase before iron forms liquid phase with increasing temperature. Thus, uranium can be hardly dissolved into the base metal, and uranium can be theoretically separated applying laser cleaning.
Second, we focused separation behaviors of powder-like uranium compounds on the coating film. In this work, a simulant specimen was prepared putting cerium oxide powders on the painted steel, because it was not appropriate to perform hot tests using actually uranium-contaminated specimens in this early stage of research. Shows similar behaviors to uranium oxide, since it has the same crystal structure (fluorite type structure). As shown in Fig.8-12, cerium peaks disappeared after laser irradiation, and iron and chromium peaks appeared. This result indicates that the laser cleaning performed in this work can remove both the cerium oxide powders and the coating film on the steel surface.
Finally, we focused the scattered direction of removed airborne dusts through the high-speed camera observation. As shown in Fig.8-13, most removed airborne dusts like fume scattered perpendicular to the specimen surface, and the direction was independent of the laser irradiation angle. Thus, the airborne dusts could be efficiently recovered from the vertical direction to the surface.
From these results, laser cleaning can be possibly applied to removing the uranium-contaminated coating film painted on steel. In future work, we conduct some decontamination tests using actually uranium-contaminated specimens.
(Ikumi Yamane)