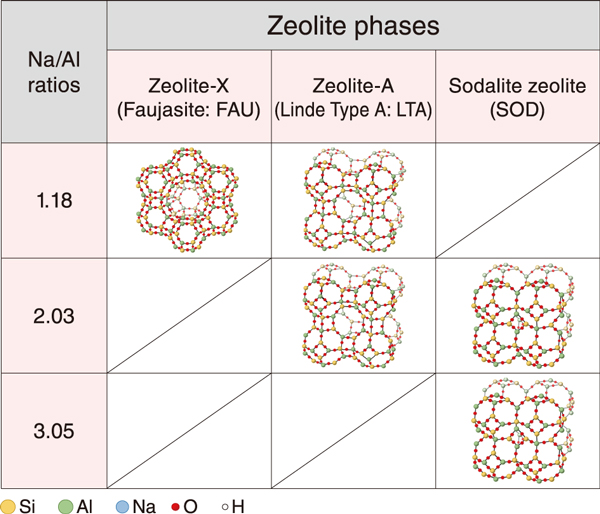
Fig.1 Types and structures of the formed zeolite phases
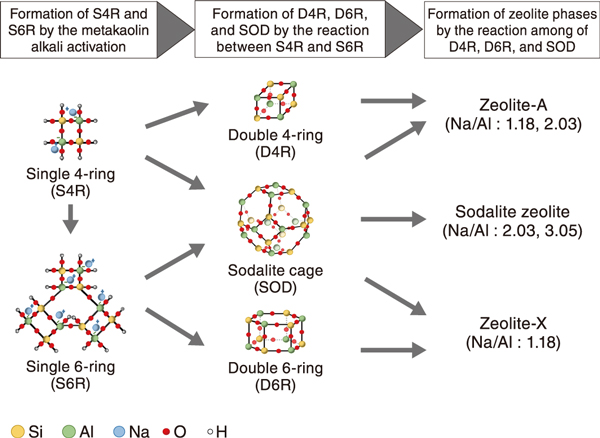
Fig.2 Possible formation pathway of zeolite phases
Radioactive wastes should be solidified in a container using solidification materials such as cements or glasses to ensure their safe disposal. Glasses immobilize radioactive nuclides in an amorphous network structure, but such treatment requires high temperatures. Cements solidify at room temperature, but their immobilization performance is less than that of glasses.
We focus on the geopolymer, which hardens at room temperature like cements and immobilizes radioactive nuclides such as cesium in a glass-like amorphous structure, as a candidate for a new solidification material. Considering the properties of the geopolymer, research and development on its application for treating radioactive wastes to stabilize radioactive nuclides or hazardous substances contained in radioactive wastes has progressed.
Geopolymers are known to alternate from amorphous structures to crystalline phases such as zeolite over time. To stabilize the radioactive and hazardous substances even after disposal, it is necessary to estimate the long-term performance of the waste forms with regard to its alternation. Therefore, the effect of the material composition of the geopolymer on the type of zeolites formed over time was investigated in this study.
Metakaolin, which has an amorphous structure composed of silicon and aluminum, was used as the raw material, and sodium hydroxide solution was used as an alkaline solution. Na/Al ratios of 1.18, 2.03, and 3.05 were applied by changing the ratios of the alkaline solution and metakaolin. After curing at 20 ℃ for up to 112 days, the crystalline phases and changes in chemical bonds in the solidified samples were examined by X-ray diffraction analysis and infrared spectroscopy, respectively.
Zeolites started to be detected after curing for 28 days. In the solidified products cured for 112 days, zeolite-X and zeolite-A were detected at a Na/Al ratio of 1.18; zeolite-A and sodalite were detected at a Na/Al ratio of 2.03; and sodalite was detected at a Na/Al ratio of 3.05 (Fig.1). It was found that the zeolite phases formed in solidified products differ depending on the Na/Al ratio. In addition, the difference in the formation pathway of zeolites with the Na/Al ratios is estimated as shown in Fig.2. based on the analysis of chemical bonds and a reference*.
In the future, the effects of the formed zeolites on the performance of the geopolymer, such as the immobilization of radioactive or hazardous substances and compressive strength, will be investigated. We will continue the research and development on solidification materials for radioactive wastes with higher performance than that of the existing materials.
(Junya Sato)
* Mohau, M. et al., A Review of the Chemistry, Structure, Properties and Applications of Zeolites, American Journal of Materials Science, vol.7, issue 5, 2017, p.196-221.