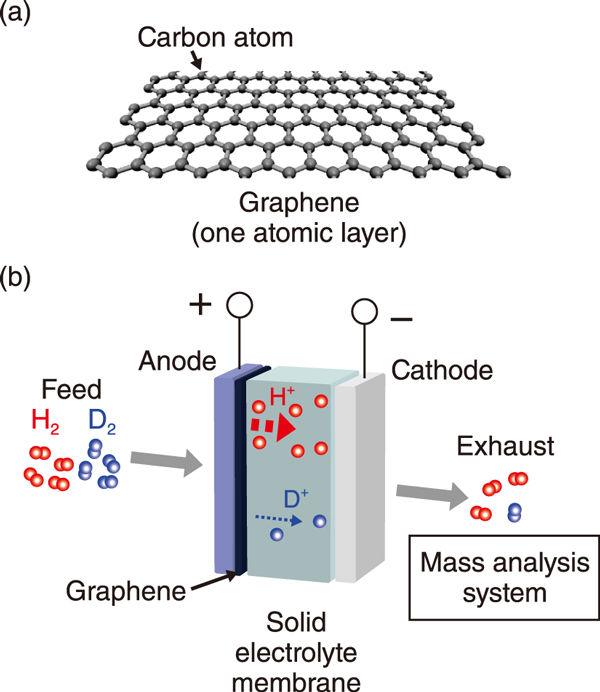
Fig.1 (a) Graphene and (b) a schematic illustration of the evaluation of the ability of graphene to separate hydrogen (H) / deuterium (D) using electrochemical method
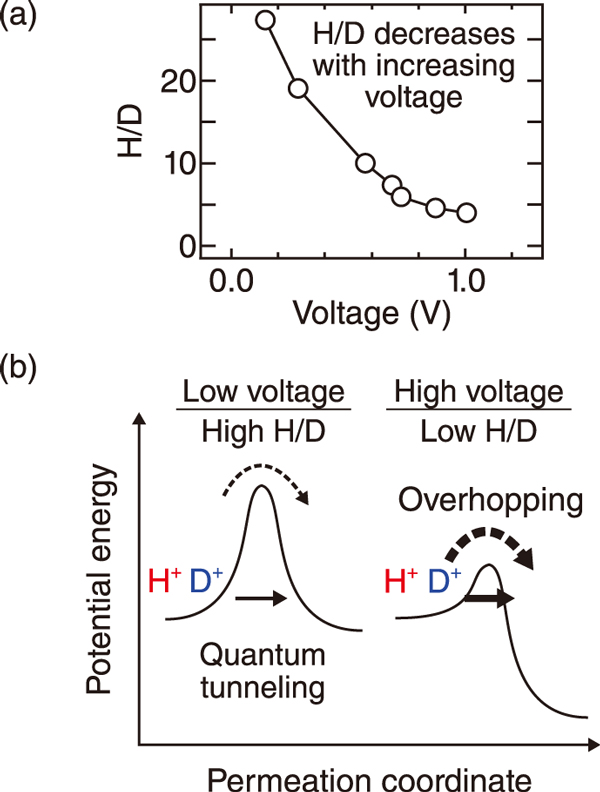
Fig.2 (a) H/D separation ability vs. voltage. (b) schematic of ion transfer through the graphene at different voltages
Deuterium (D), which is a stable isotope of hydrogen (H), is an essential material in industrial fields, such as semiconductor, organic electroluminescence and pharmaceutical industries, and is regarded a future energy source for nuclear fusion. D is obtained by concentrating and separating trace amounts of D present in hydrogen. However, poor H/D separation ability and the need to achieve a cryogenic temperature for the process result in a high production cost.
Graphene is a one-atom-thick sheet with a honeycomb structure of carbon atoms bonded in hexagonal patterns (Fig.1(a)). Current studies have suggested that light hydrogen ions (H+) permeate more selectively than heavy deuterium ions (D+) through the “pores” in the central part of the honeycomb structure, indicating high H/D separation ability at room temperature. Hence, graphene has garnered much attention as a low-cost deuterium-enrichment material. However, because of the challenging experimental setup, the mechanism of the separation ability remained unclear. In this study, we utilized an electrochemical reaction system with a solid electrolyte membrane to elucidate the separation mechanism (Fig.1(b)). When a voltage is applied between the electrodes, H2 and D2 in a gas mixture supplied to the anode are ionized as H+ and D+ and are released at the graphene side. The ions sifted with graphene flow to the cathode and then are converted to gaseous forms of hydrogen isotopes. The gas analysis showed that at the cathode, more H2 was released than D2, demonstrating the H/D separation ability of graphene. Further, the higher voltage, the lower was the separation ability (Fig.2(a)). Theoretical analysis revealed that the high H/D ability originates from the quantum tunneling of the ions through the graphene (Fig.2(b)). Lighter H+ ions can permeate through graphene more easily than D+, leading to the high H/D ability. The bias dependence of H/D originates from the transition from the quantum tunneling regime to the classical overbarrier regime for the ion transfer through the graphene because of the gradual reduction of the barrier height of graphene.
In conclusion, we demonstrated that the high H/D separation ability of graphene originates from the quantum tunneling effect. This finding will contribute to the development of economical deuterium-enrichment systems using graphene.
This research was supported by JSPS KAKENHI Grant-in-Aid for Scientific Research (B) (JP21H01751) “Control of Hydrogen Isotope Separation by Hydrogen Ion Permeable Hetero Electrode Interface.”
(Satoshi Yasuda)