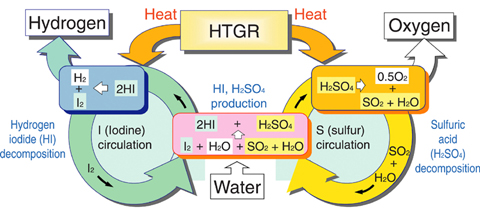
Fig.7-19 IS process
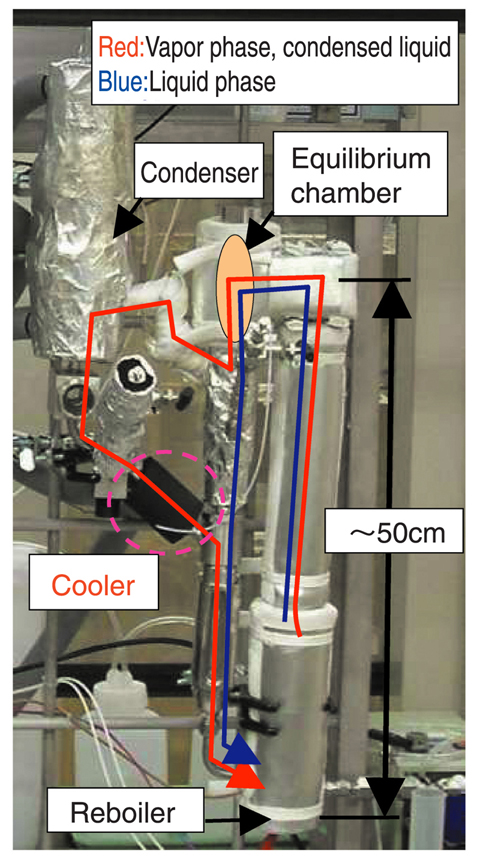
Fig.7-20 VLE measurement device
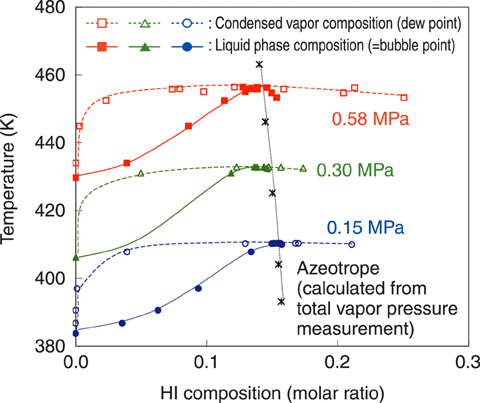
Fig.7-21 VLE data of HI+H2O mixture
We are conducting R&D of thermochemical iodine-sulfur (IS) process, which can produce massive amounts of hydrogen from water without CO2 emission, using the heat supplied by High Temperature Gas-cooled Reactors (HTGR). Fig.7-19 shows the scheme of IS process. Water (H2O) reacts with iodine (I2) and sulfur dioxide (SO2) to produce hydrogen iodide (HI) and sulfuric acid (H2SO4), which are then thermally decomposed, producing hydrogen and oxygen, respectively. One of the difficult technical requirements of the process is efficient separation of HI from HI+I2+H2O mixture. Distillation under elevated pressure is the promising candidate. However, little is known about the relevant vapor-liquid equilibrium (VLE).
Therefore, we have started the acquisition of VLE data. Our first target was the VLE of hydriodic acid, the two component system of HI+H2O. At first, the materials for measurement devices which can be used in the highly corrosive HI+H2O solution were screened. Next, using the selected materials, a measurement device was constructed as shown in Fig.7-20. Here, in order to avoid the re-boiling of vapor condensate in the recycle line due to the large difference of boiling points of the vapor condensate and the liquid phase, a cooling system was added.
Fig.7-21 shows the obtained VLE data. HI+H2O is known to exhibit azeotropy, where compositions of the two phases of HI+H2O are identical. The measured azeotropic points were well in accord with those estimated from the former data of total vapor pressure measurement, which demonstrated the reliable accuracy of the developed measurement system. The equilibrium composition data of vapor and liquid phases newly acquired in the present study will enable precise design of the distillation column with smaller redundancy.
The acquisition of high pressure VLE data of three component system of HI+I2+H2O system is under study. The VLE database will enable the compact and efficient design of the key separation apparatus of IS process.