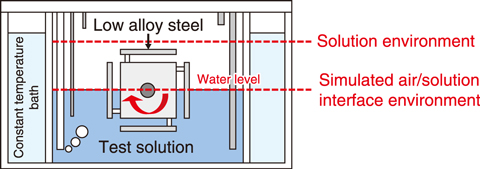
Fig.4-6 Simulated air/solution interface and corrosion environment
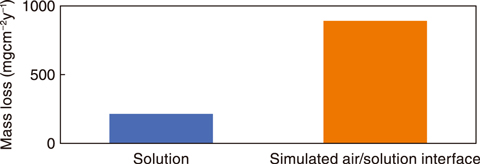
Fig.4-7 Corrosion acceleration at the simulated air/solution interface
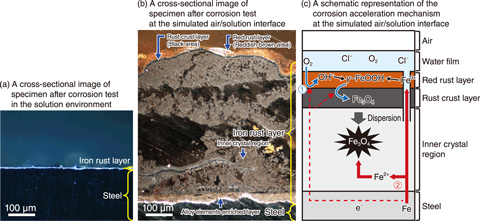
Fig.4-8 Cross-sectional images of specimens after corrosion tests (a) in the solution environment and (b) at the simulated air/solution interface; and (c) a schematic representation of the corrosion acceleration mechanism at the simulated air/solution interface
Units 1–3 of the TEPCO’s Fukushima Daiichi NPS (1F) have been injected with cooling water; internal investigations of the reactor have confirmed that low-alloy steels (steels containing ≤10% of alloying elements) are exposed to an air/solution interface. Previous studies have shown that the corrosion rate of carbon steel at the air/solution interface is increased by the formation of a liquid film on the carbon steel, as the film accelerates the oxygen reduction reaction. However, the corrosion rate of low-alloy steels used in the 1F reactor pressure vessel (RPV) at the air/solution interface have not yet been clarified.
Therefore, corrosion tests of low-alloy steel were performed in a solution environment and at a simulated air/solution interface using the test apparatus shown in Fig.4-6. The results indicated that the corrosion rate of low-alloy steel at the air/solution interface was more than four times faster than that in the solution environment (see Fig.4-7).
Cross-sectional observations of the rust layer were then performed to clarify the mechanism behind this acceleration; the resulting images are shown in Figs.4-8(a) and (b). Although a red rust layer was formed in the solution environment, it was peeled off during the process of pulling up from the solution, leaving only a thin layer of iron rust. On the other hand, cross-sectional analysis indicated that the rust layer formed at the simulated air/solution environment had a red rust layer consisting of γ-FeOOH, a rust crust layer consisting of Fe3O4, an inner crystal region composed of crystalline Fe3O4, and an alloy-elements-enriched layer at the rust–steel interface. Only the inner crystal region became thicker during the growth of the iron rust layer.
A schematic of the accelerated corrosion mechanism of the low-alloy steel at the simulated air/solution interface is shown in Fig.4-8(c). Here, the oxygen reduction reaction (① : 1/2O2 + H2O + e− → 2OH−), which controls corrosion, occurs in the rust crust layer near the liquid film that is in contact with the air. The electrical connection between the crust layer and steel was confirmed through the inner crystal region; this suggests that electrons generated by the iron dissolution reaction ( ② : Fe → Fe2+ + 2e− ) are supplied to the crust layer. The oxygen reduction reaction thus likely occurs in the crust layer despite the thickening of the inner crystal region. Meanwhile, no crust layer was formed in the solution environment, due to the low supply of oxygen via diffusion. Therefore, a slower corrosion rate is expected in the solution environment, because the accelerated corrosion, contributed to by the rust crust layer, does not occur.
This study shows that the formation of the crust layer causes the accelerated corrosion of low-alloy steel at the air/solution interface. Future work will aim to clarify the effects of oxygen concentration and seawater composition on the corrosion rate and mechanism, which will contribute to ensuring the long-term integrity of the 1F RPV.
(Kyohei Otani)