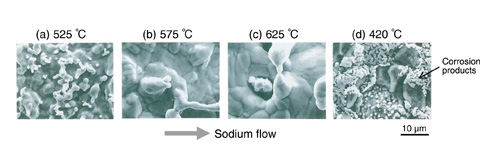
Fig.13-22 Corrosion of 304 SS exposed in flowing sodium for 82000 h
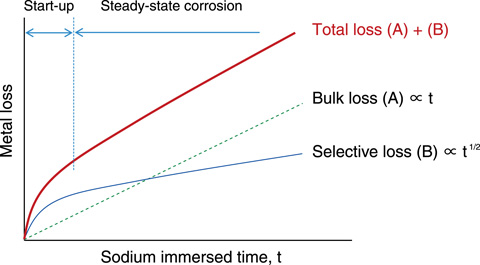
Fig.13-23 Schematic diagram of sodium corrosion characteristics
For sodium-cooled fast breeder reactors (FBRs), the effects of a sodium environment on the corrosion and mechanical strength properties of structural materials have to be evaluated in order to maintain the material integrity throughout the design lifetime.
Because the oxides that form on the materials are easily reduced by sodium, a liquid-phase metal (sodium) comes into direct contact with solid-phase metals (structural materials). When this happens, because of the difference in the chemical potential depending on the temperature and time, either the alloy elements dissolve from the metal surface into the sodium or the inverse process (i.e., deposition) occurs. These phenomena would be essentially controlled by the saturation solubility of the alloy elements and the oxygen concentration in sodium.
In a non-isothermal sodium circulation loop such as that in FBRs, the alloy elements dissolve in the higher-temperature region, and the dissolved elements are deposited in the lower-temperature region because of supersaturation (Fig.13-22). Start-up corrosion is caused by the selective dissolution of alloy elements such as nickel and chromium into sodium. In contrast, steady-state corrosion is dominated by the corrosion reaction of iron (Fig.13-23). Furthermore, because the carbon contained in steels plays an important role in maintaining the material’s strength properties, considerable emphasis should be placed on the decarburization and carburization phenomena in sodium. In a bimetallic sodium circulation loop consisting of stainless and ferritic steels, because of the difference in carbon activity between the two materials, decarburization occurs in ferritic steel, which has higher carbon activity, whereas carburization occurs in stainless steel.
In addition, because FBRs are used at higher temperatures than light water reactors, it is necessary to understand the creep properties in addition to the tensile and fatigue properties. Furthermore, the damage caused by the superposition of creep and fatigue becomes dominant in response to repetitive loading due to thermal stress. Therefore, it is also important to understand the creep-fatigue strength properties. On the other hand, it is understood that the effects of decarburization/carburization and sodium corrosion of materials are not a dominant factor in impurity-controlled sodium.
This knowledge has been summarized to describe the environmental effects of high-temperature sodium and is reflected in the material strength standard for the design of sodium-cooled FBRs.